vaccine delivery system pdf
On January 28 2021 the VAC delivered its final recommendations for vaccine phases that would follow. This committee makes recommendations on use of vaccines approved by the FDA.
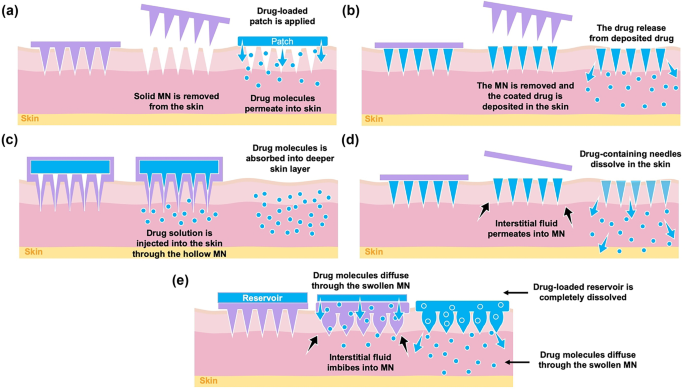
Enhancement Strategies For Transdermal Drug Delivery Systems Current Trends And Applications Springerlink
The loss of vaccine effectiveness due to cold chain exposures to adverse conditions is cumulative permanent and irreversible.
. The PfSPZ vaccine is a candidate malaria vaccine developed by Sanaria using radiation-attenuated sporozoites to elicit an immune response. Using Vaccine Adverse Event Reporting System data this study examined reports of LRTIs in infants 6-15 weeks old who received one of two rotavirus vaccines Rotarix or RotaTeq in addition to either the 7-valent PCV7 or 13-valent PCV13 pneumococcal conjugate vaccine. COVID-19 Vaccine Religious Exception Request Form pdf.
Reichmuth AM Oberli MA Jaklenec A et al. A second dose inventory management system. Synchronization of our vaccine shipments with the delivery of an ancillary kit that contains supplies required to administer the vaccine and.
Incomplete applications may be delayed. This pharmaceutical conference 2022 is a 3 day event with symposiums and discussions on Pharmaceutics and Drug Delivery Systems. Click for Vaccine Locations.
Failure to adhere to vaccine handling and cold chain requirements may reduce vaccine potency resulting in a lack of protection against COVID- 19 andor increased local reactions at the site of the vaccine administration. The gene is then expressed in APCs and the resulting protein product is processed into epitopes. A practical reference that facilitates and enhances the global delivery of quality immunization healthcare to Department of Defense DoD beneficiaries and employees.
Vaccines also known as immunizations inject a weakened form of a. The additional doses make that response stronger particularly the last one which fortifies the memory response. Public health emergencies can affect our families communities and businesses.
It takes about two weeks after the first dose of vaccine for the immune system to generate an immune response. Modified nucleotides capable of escaping immune system surveillance have been introduced in the mRNA. Since tumors often evolve mechanisms to suppress the immune system.
Andia Alamy Stock Photo. Vaccine Adverse Event Reporting System VAERS. Herein we reported a lymph-nodetargeting mRNA vaccine based on lipid nanoparticles named 113-O12B for cancer immunotherapy.
A cancer vaccine is a vaccine that either treats existing cancer or prevents development. The Idaho HAN system is an automated system designed to rapidly deliver time-critical health-related information. Our document repository has a full list of PDF document downloads.
The EUA fact sheets for age 6 through 11. The benefits of vaccines far outweigh the risks. The combination of OWS logistics expertise coupled with Pfizers deep manufacturing and distribution expertise provides a solid foundation for success.
Clinical trials have been promising with trials in Africa Europe and the US protecting over 80 of volunteers. It has been subject to some criticism regarding the ultimate feasibility of large-scale production and delivery in Africa since. Seventh Edition of Pharma Conference 2022 is making its mark in the world of researchers related to Drug Delivery Systems and Drug Delivery Conferences occurring as Online Event during September 8-10 2022.
Fact Sheets for Moderna COVID-19 Vaccines Age 6 through 11 years for primary series and age 18 for 05 mL booster dose dark blue cap purple border This single formulation has separate EUA fact sheets for its use as a primary series dose in children age 6 through 11 years and for its use as a booster dose in adults 18. To apply for a vaccine ordering account please complete the New application for a Onelink vaccine ordering account all providers external site and follow the instructions for submitting your completed application. The Defense Health Agency Immunization Healthcare Division DHA-IHD publishes the Immunization Tool Kit based on national recommendations evidenced-based peer-reviewed.
Meetings during the month of January 2021 and held three optional information sessions to discuss topics related to vaccine delivery. Copy link to page Download PDF. Notably 113-O12B can efficiently deliver both a full-length.
To order COVID-19 vaccines please use the COVID-19 Vaccination Administration System external site. Delivery of the gene is particularly challenging for this type of vaccine. This overview focuses on vaccine research how vaccines are licensed and how we make sure vaccines are safe.
Security measures comply with Health Insurance Portability and Accountability Act HIPAA and Kansas statutes. Access is limited to individuals and entities that either provide immunization services or are required to ensure that persons are immunized. The global Vaccines Market size accounted for 3806115 Million in 2021 and is expected to reach 7212961 Million by 2031 registering a CAGR of 66 from 2022 to 2031.
The ACIP also advises whether the new vaccine should be added to the Recommended Child and Adolescent Immunization Schedule. After reading this article you should be able to. Even after a vaccine is approved and recommended for use the safety and effectiveness of the vaccine continues to be monitored by the CDC and FDA.
As science continues to advance we strive to develop safer vaccines and improve delivery to protect ourselves against disease more effectively. The targeted delivery of the mRNA vaccine elicits robust CD8 T cell responses exhibiting excellent protective and therapeutic effects on B16F10 melanoma. MRNA vaccine delivery using lipid nanoparticles.
Covid 19 Vaccine Brand Hesitancy And Other Challenges To Vaccination In The Philippines Plos Global Public Health
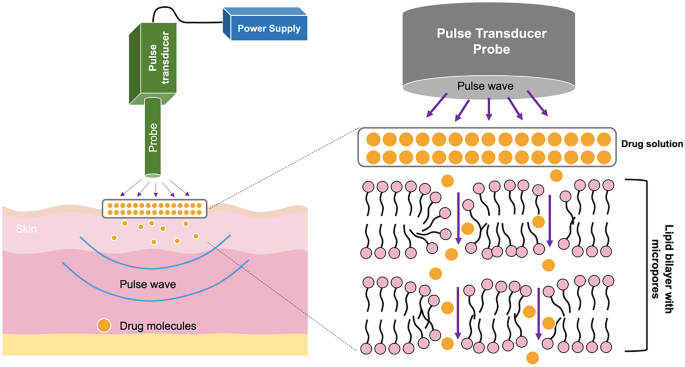
Enhancement Strategies For Transdermal Drug Delivery Systems Current Trends And Applications Springerlink
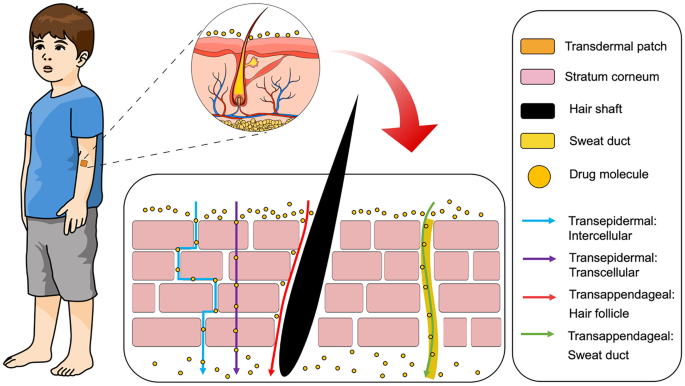
Enhancement Strategies For Transdermal Drug Delivery Systems Current Trends And Applications Springerlink

Types Of Vaccines Infographics Epidemiology Covid 19 Response Corps

Novel Drug Delivery Systems Sciencedirect

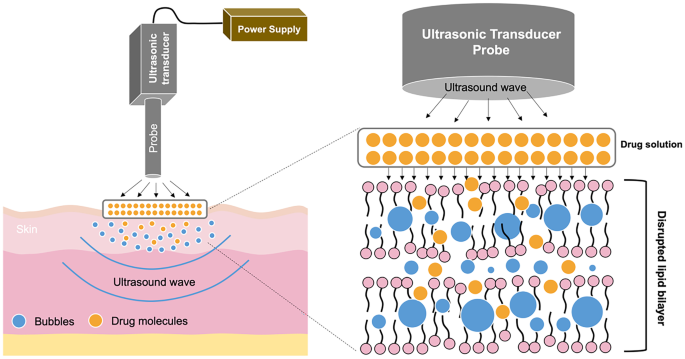
Enhancement Strategies For Transdermal Drug Delivery Systems Current Trends And Applications Springerlink

Lipid Nanoparticles From Liposomes To Mrna Vaccine Delivery A Landscape Of Research Diversity And Advancement Acs Nano
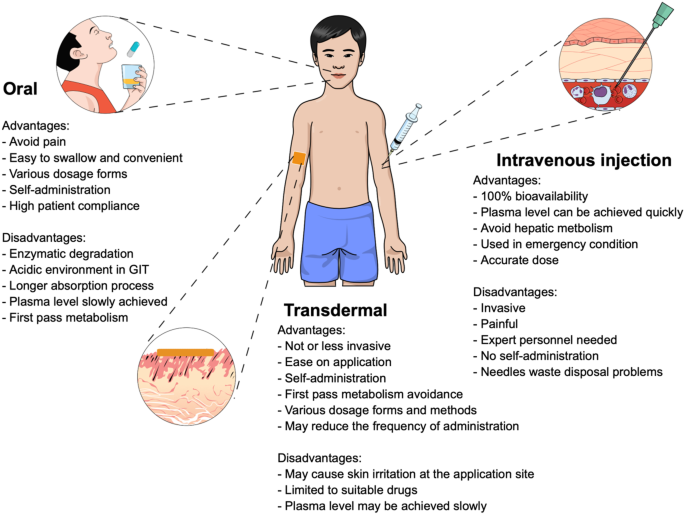
Enhancement Strategies For Transdermal Drug Delivery Systems Current Trends And Applications Springerlink
Covid 19 Vaccine Brand Hesitancy And Other Challenges To Vaccination In The Philippines Plos Global Public Health
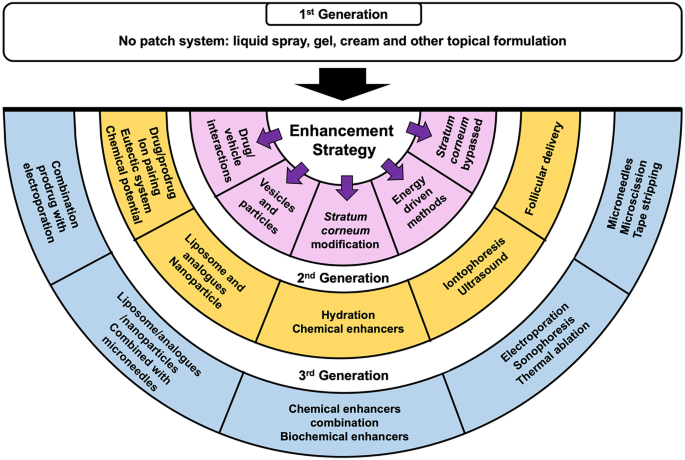
Enhancement Strategies For Transdermal Drug Delivery Systems Current Trends And Applications Springerlink

Lipid Nanoparticles From Liposomes To Mrna Vaccine Delivery A Landscape Of Research Diversity And Advancement Acs Nano
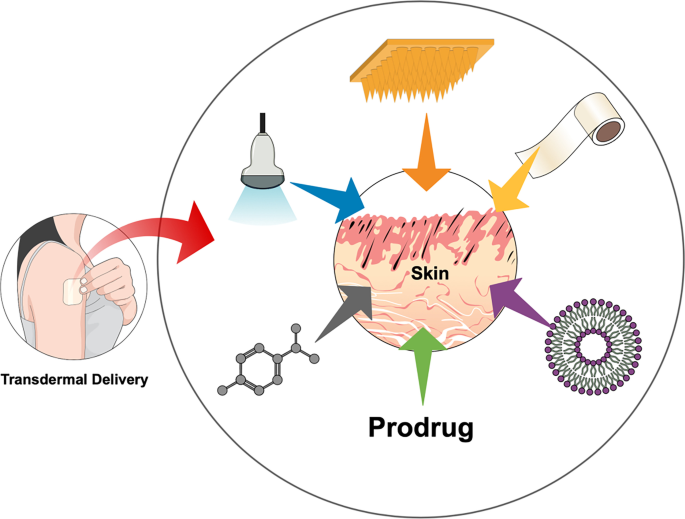
Enhancement Strategies For Transdermal Drug Delivery Systems Current Trends And Applications Springerlink